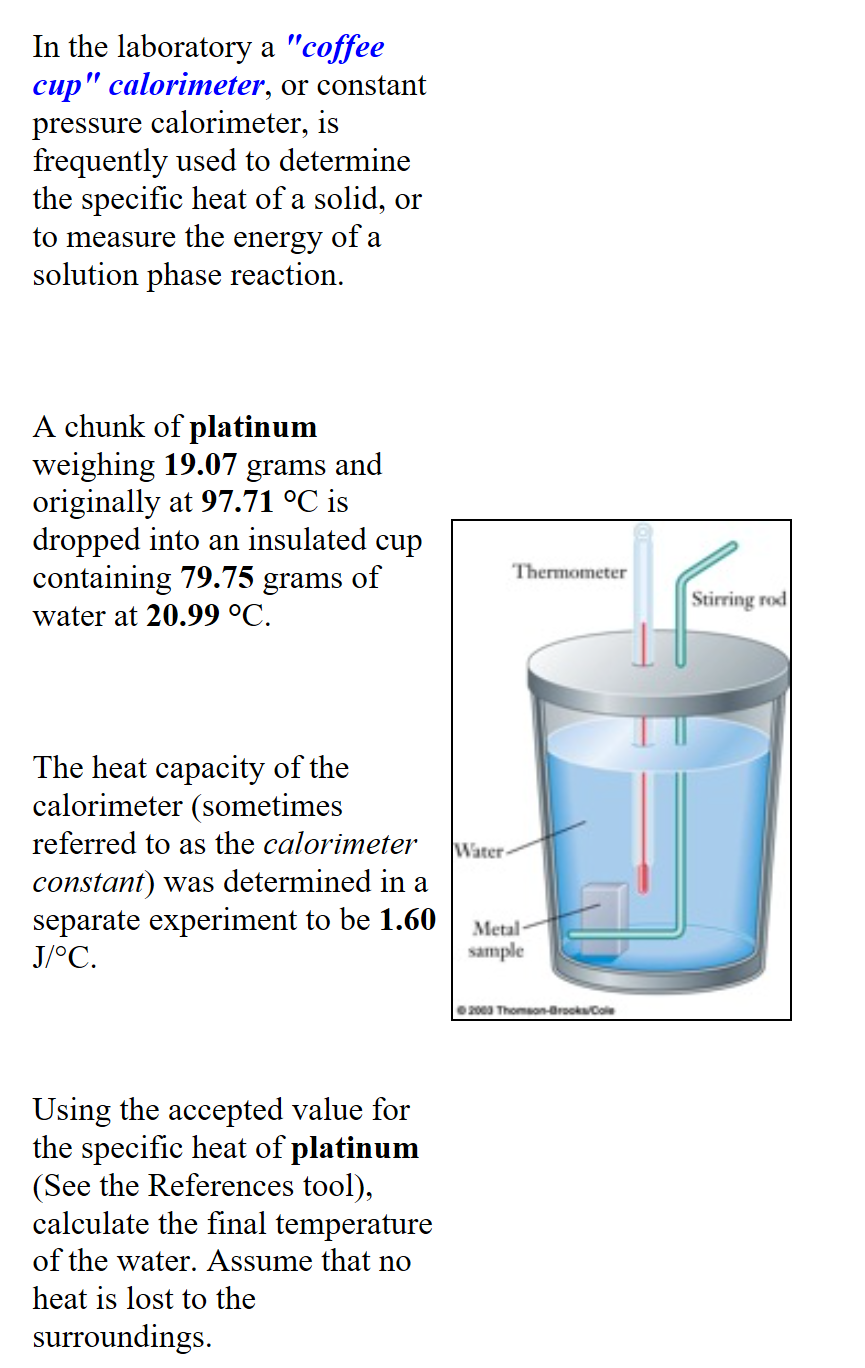
A Calorimeter Is Used To Measure What . A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists of a metallic vessel made of materials which are good. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Apply the first law of thermodynamics to calorimetry.
from www.bartleby.com
For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Apply the first law of thermodynamics to calorimetry. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for heat measurements necessary for calorimetry. It mainly consists of a metallic vessel made of materials which are good. For example, when an exothermic.
Answered In the laboratory a "coffee cup"… bartleby
A Calorimeter Is Used To Measure What It mainly consists of a metallic vessel made of materials which are good. Apply the first law of thermodynamics to calorimetry. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For example, when an exothermic. It mainly consists of a metallic vessel made of materials which are good. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From www.animalia-life.club
Food Calorimeter A Calorimeter Is Used To Measure What Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For example, when an exothermic. A calorimeter is a device. A Calorimeter Is Used To Measure What.
From www.deltaed.co.nz
Calorimeter Joules w Electric Heating Delta Educational A Calorimeter Is Used To Measure What Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. It mainly consists of a metallic vessel made of materials which are good. For example, when an exothermic.. A Calorimeter Is Used To Measure What.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation A Calorimeter Is Used To Measure What For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. It mainly consists of a metallic vessel made of materials which are good. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow. A Calorimeter Is Used To Measure What.
From saylordotorg.github.io
Calorimetry A Calorimeter Is Used To Measure What For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimeters are used. A Calorimeter Is Used To Measure What.
From chemistrytalk.org
Calorimetry ChemTalk A Calorimeter Is Used To Measure What For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction.. A Calorimeter Is Used To Measure What.
From www.education.com
Calorimetry Bomb Calorimeter Experiment A Calorimeter Is Used To Measure What Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. It mainly consists of a metallic vessel made of materials which are good. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry.. A Calorimeter Is Used To Measure What.
From people.chem.umass.edu
to Adobe GoLive 6 A Calorimeter Is Used To Measure What Apply the first law of thermodynamics to calorimetry. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. A Calorimeter Is Used To Measure What.
From www.indiamart.com
Stainless Steel Single Phase Automatic Bomb Calorimeter, Size 325 x A Calorimeter Is Used To Measure What Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It mainly consists. A Calorimeter Is Used To Measure What.
From users.highland.edu
Calorimetry A Calorimeter Is Used To Measure What A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. A Calorimeter Is Used To Measure What.
From bettyelevisxo.blob.core.windows.net
Fundamentals Of Calorimetry Lab A Calorimeter Is Used To Measure What Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It mainly consists of a metallic vessel made of materials which are good. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter. A Calorimeter Is Used To Measure What.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle A Calorimeter Is Used To Measure What Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. Apply the first law. A Calorimeter Is Used To Measure What.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 A Calorimeter Is Used To Measure What It mainly consists of a metallic vessel made of materials which are good. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science. A Calorimeter Is Used To Measure What.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors A Calorimeter Is Used To Measure What A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter is a device used for heat measurements necessary for calorimetry. It mainly consists of a metallic vessel made of materials which are good.. A Calorimeter Is Used To Measure What.
From www.animalia-life.club
Food Calorimeter A Calorimeter Is Used To Measure What A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Apply the first law of thermodynamics to calorimetry. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron). A Calorimeter Is Used To Measure What.
From labsuppliesusa.com
Calorimeter Electric KLM Bio Scientific A Calorimeter Is Used To Measure What For example, when an exothermic. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimeter, device for measuring the heat developed during a. A Calorimeter Is Used To Measure What.
From www.thoughtco.com
Calorimeter Definition in Chemistry A Calorimeter Is Used To Measure What A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. For example, when an exothermic. A calorimeter is a device used for heat measurements necessary for calorimetry. In chemistry and thermodynamics, calorimetry (from latin calor. A Calorimeter Is Used To Measure What.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses A Calorimeter Is Used To Measure What For example, when an exothermic. In chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the science or act of measuring. It mainly consists of a metallic vessel made of materials which are good. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters are. A Calorimeter Is Used To Measure What.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 A Calorimeter Is Used To Measure What A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a. A Calorimeter Is Used To Measure What.